Mellon Lab
- Research
- Lab Members
- Publications
- Contact
We are interested in how the brain controls reproduction through the neuroendocrine system with emphasis on development, hormone signaling, gene expression, circadian rhythm, and mouse models of human disease.
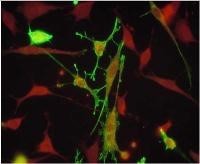 Estrogen, testosterone, and progesterone regulate hypothalamic GnRH neurons. Neuropeptides such as Kisspeptin, Neurokinin B, and Dynorphin directly impact GnRH synthesis and release. The interactions of these signaling cascades produce differential regulation of the synthesis and secretion of GnRH leading to fertility, the estrous cycle, puberty, and menopause. We utilize in vitro, cell culture, and in vivo genetic mouse models to understand the integration and interactions of signaling these cascades.
Estrogen, testosterone, and progesterone regulate hypothalamic GnRH neurons. Neuropeptides such as Kisspeptin, Neurokinin B, and Dynorphin directly impact GnRH synthesis and release. The interactions of these signaling cascades produce differential regulation of the synthesis and secretion of GnRH leading to fertility, the estrous cycle, puberty, and menopause. We utilize in vitro, cell culture, and in vivo genetic mouse models to understand the integration and interactions of signaling these cascades.
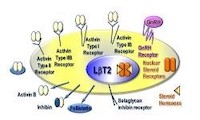 Estrogen, testosterone, and progesterone regulate pituitary luteinizing hormone (LH) and follicle-stimulating hormone (FSH) in concert with pulsatile GnRH from the hypothalamus and autocrine activin and other growth factors. The interactions of these signaling cascades produce differential regulation of the synthesis and secretion of LH versus FSH from the gonadotrope cell of the pituitary leading to fertility, the estrous cycle, puberty, and menopause. We utilize in vitro, cell culture, and in vivo genetic mouse models to understand the integration and interactions of these signaling cascades.
Estrogen, testosterone, and progesterone regulate pituitary luteinizing hormone (LH) and follicle-stimulating hormone (FSH) in concert with pulsatile GnRH from the hypothalamus and autocrine activin and other growth factors. The interactions of these signaling cascades produce differential regulation of the synthesis and secretion of LH versus FSH from the gonadotrope cell of the pituitary leading to fertility, the estrous cycle, puberty, and menopause. We utilize in vitro, cell culture, and in vivo genetic mouse models to understand the integration and interactions of these signaling cascades.
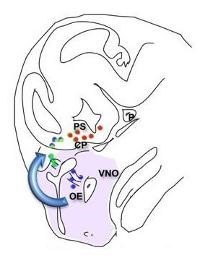 The GnRH neurons are born in the olfactory placode at e11 in the mouse, only 800 cells total. They migrate up to the olfactory bulb and back down to the hypothalamus. Defective migration or the accompanying differentiation of GnRH neurons is a cause of human infertility. We study both mouse and cell culture models of differentiation and migration, elucidating roles for homeodomain proteins, neuropeptides and their receptors, chromatin modifications, and transcriptional co-repressors and co-activators and determining their mechanisms of action.
The GnRH neurons are born in the olfactory placode at e11 in the mouse, only 800 cells total. They migrate up to the olfactory bulb and back down to the hypothalamus. Defective migration or the accompanying differentiation of GnRH neurons is a cause of human infertility. We study both mouse and cell culture models of differentiation and migration, elucidating roles for homeodomain proteins, neuropeptides and their receptors, chromatin modifications, and transcriptional co-repressors and co-activators and determining their mechanisms of action.
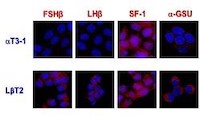 Cell fate of the gonadotrope within the pituitary is determined by internal genetic programs coupled with external cues. We study both mouse and cell culture models of differentiation and migration, elucidating roles for homeodomain proteins, neuropeptides and their receptors, chromatin modifications, and transcriptional co-repressors and co-activators and determining their mechanisms of action.
Cell fate of the gonadotrope within the pituitary is determined by internal genetic programs coupled with external cues. We study both mouse and cell culture models of differentiation and migration, elucidating roles for homeodomain proteins, neuropeptides and their receptors, chromatin modifications, and transcriptional co-repressors and co-activators and determining their mechanisms of action.
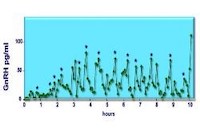 Mutation of circadian rhythm genes leads to profound infertility, absent puberty and estrous cycles, and lack of pulsatile GnRH secretion. Studying the actions of the “clock” genes in an immortalized hypothalamic neuron that secretes GnRH in the physiological pulsatile pattern, along with reproductive function in genetically modified mice, will reveal the molecular mechanisms for specifying the rhythms of the reproductive cycle and pulsatile depolarization of the hypothalamic neurons. Steroid hormone feedback contributes to these rhythms and regulates the estrous cycle, puberty, and reproductive senescence. Thus, studies with targeted knock-out of nuclear receptors and clock genes in mice coupled with studies of cells in vitro can reveal the underlying mechanisms of reproductive rhythms.
Mutation of circadian rhythm genes leads to profound infertility, absent puberty and estrous cycles, and lack of pulsatile GnRH secretion. Studying the actions of the “clock” genes in an immortalized hypothalamic neuron that secretes GnRH in the physiological pulsatile pattern, along with reproductive function in genetically modified mice, will reveal the molecular mechanisms for specifying the rhythms of the reproductive cycle and pulsatile depolarization of the hypothalamic neurons. Steroid hormone feedback contributes to these rhythms and regulates the estrous cycle, puberty, and reproductive senescence. Thus, studies with targeted knock-out of nuclear receptors and clock genes in mice coupled with studies of cells in vitro can reveal the underlying mechanisms of reproductive rhythms.
 Prenatal exposure to very low levels of androgens, environmental estrogen mimetics, or high-fat diet causes female precocious puberty, irregular reproductive cycles, and other hallmarks of reproductive disorders. In addition, genetic models for inherited defects in neuronal migration and brain development allow the study of the genetic basis of infertility. Projects address the molecular mechanisms by which prenatal exposure to environmental hormones and endocrine disruptors can generate reproductive disorders. In addition, mouse models of genetic disorders such as Prader-Willi Syndrome, Polycystic Ovary Syndrome, and Hypothalamic Hypogonadism allow investigation of the mechanisms of neuronal disorganization and dysfunction that result in these syndromes.
Prenatal exposure to very low levels of androgens, environmental estrogen mimetics, or high-fat diet causes female precocious puberty, irregular reproductive cycles, and other hallmarks of reproductive disorders. In addition, genetic models for inherited defects in neuronal migration and brain development allow the study of the genetic basis of infertility. Projects address the molecular mechanisms by which prenatal exposure to environmental hormones and endocrine disruptors can generate reproductive disorders. In addition, mouse models of genetic disorders such as Prader-Willi Syndrome, Polycystic Ovary Syndrome, and Hypothalamic Hypogonadism allow investigation of the mechanisms of neuronal disorganization and dysfunction that result in these syndromes.